Re-educating our immune system to tackle chronic disease
Groundbreaking research out this week is taking a novel approach to help chronic disease
Our immune system is incredibly powerful with a host of tricks that enable us to fight off an incredible array of infections. However, our immune system can also go rogue and turn against us by attacking our own cells and tissues - just as if we were an infectious threat. This is exactly what happens in autoimmune diseases which include conditions such as rheumatoid arthritis and inflammatory bowel diseases such as Crohn’s disease. Autoimmune conditions are incredibly common affecting around 5-8% of us. They are also incredibly tricky to manage and treat.
Most treatments for autoimmunity try to tackle the inflammation which can relieve many of the symptoms. But, these treatments don’t necessarily deal with the root causes of the autoimmune condition - so they are not a cure. Nonetheless, there have been some amazing innovations including immunotherapies that block the action of proinflammatory products like anti-TNF therapy. However, immunotherapies are expensive, are not suitable for everyone, can have substantial toxicities and do not represent a cure. Sadly, even with immunotherapies, many patients suffering from autoimmune diseases can relapse.
Many, but not all, autoimmune diseases are characterised by the production of antibodies that recognise our own tissues or cells- we call these autoantibodies. Autoantibodies can be one of the main culprits causing the damage in autoimmune conditions. Researchers asked if they could tackle these troublesome autoantibodies at source- the antibody producing B cells- and research just published show the early results from a clinical trial using CAR T cells.
CAR T cells
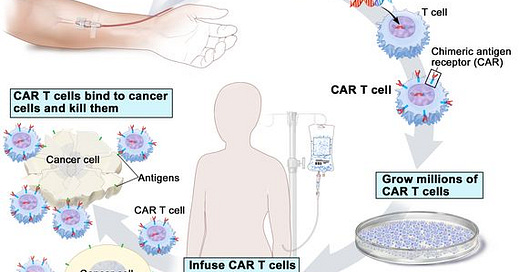
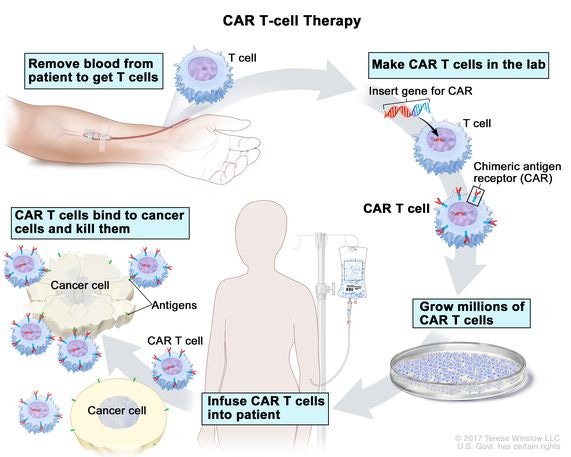
First though-a brief explainer on what we mean by CAR T cells. CAR T cells are a type of personalised cell therapy that is increasingly being used in the treatment of some aggressive blood cancers including lymphoma and leukemia. They are a form of “living drug” made by collecting a type of white blood cell called a T cell from the patient. The T cells are then engineered in a lab to produce proteins on their surface called chimeric antigen receptors, or CARs (hence the name CAR T cells). Chimeric antigen receptors are basically a fancy way of saying that the T cells are designed to recognise specific targets- for cancer therapy these will be unique features of the patient’s own cancer cells. The T cells are then grown up in the lab to get lots of T cells which can then be infused back into the patient. Once there, the CAR T cells act like ninja warriors- looking for the cancer cells and destroying any cells with the features that the cells have been engineered to recognise.
CAR T cell therapies for autoimmune disease?
For autoimmune diseases where autoantibodies cause much of the damage, CAR T cells have been explored that would target the antibody producing B cells. This is done by targeting a receptor expressed on B cells called CD19. CD19-targeted CAR-T therapies have been explored for treating autoimmune diseases such as lupus with early promising results. However, personalised medicines like these are very expensive and time consuming. Ideally, there would be much more scalable product that was suitable for multiple people -an accessible “off-the-shelf “ therapy. This is not easy though because of the risk of generating a transplant rejection reaction. Its for this very reason that we currently need to use the patient’s own cells. In transplant rejection reactions, our immune system perceives the donor cells as a threat because they are too unlike our own cells. The immune system will then try to eliminate the new cells and cause damaging inflammation. Gene editing tools such as CRISPR are one approach that has been investigated in a new study. What the researchers did was gene edited the T cells so they didn’t express some of the features that could trigger transplantation rejection such as HLA. The CAR T cells were also engineered to recognise the B cell target CD19 .
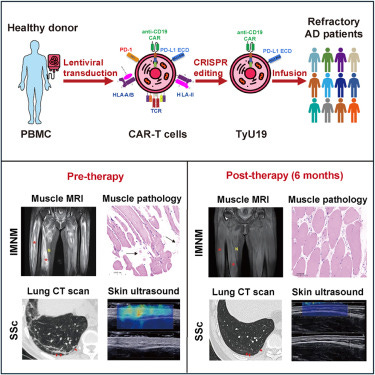
Three patients with autoimmune diseases that were not amenable to normal treatments were given the CAR T cells. The infused cells targeted the B cells with a complete loss of B cells seen within 2 weeks of treatment. The loss of B cells meant no more autoantibodies were being made which ultimately resulted in a marked reduction in the levels of damaging inflammatory products known as cytokines (lasting 6 months). The CAR T cells survived for over 3 months in the patients too suggesting a reasonable durability of the treatment. The best news for the patients was that they had massive improvements in their symptoms.
This is really exciting, but its still early days. Wiping out our B cells is not a trivial undertaking and our B cells are vital in defence against infections. So we could leave people vulnerable to infections. This also is not a cheap approach and we need to know much more about durability of the results, whether patients would require further treatment and the long term safety and efficacy if so. Many people do respond well to the current range of autoimmune treatments and so such a radical treatment would not be suitable for all.
However, for those that do not respond to standard treatments and have severe refractory autoimmune disease, this type of approach using engineered CAR T cells could be a gamechanger.



Been out of the lab for a few years now, but I was still pipette in hand when CAR T was starting making a big splash. I honestly think the sky is the limit for CAR T therapy, progress has been so rapid in the last decade! I'd love to see one of two things happen: figure out how to have an immortalised cell line that works like a 'universal donor' and then just CRISPR away with that OR find a way to retain the benefits of immortalised CAR T but as a cell-free approach. For all I know all this stuff has been done already (sorry if you've linked to it). I also reckon there's huge potential for anti-aging treatment too... If that doesn't get the big bucks rolling in, nothing will!
Thank you so much for this and the explanation so that non-scientists like myself can understand. We have the scientists we just need to fund their research, it is so important!