Our immune system has evolved to deal with a plethora of pathogens and has many tricks up its sleeves to precisely target a whole range of pathogens. Immune cells can also recognise tumour cells and our T cells are really important for eradicating cancerous cells. But tumour cells can be tricky customers for immune recognition and killing.
One reason for this is that our T cells are trained from an early age to not react to our own cells and tissues (this is called immune tolerance). And here’s the thing; a tumour is essentially our own cells gone rogue - so that in of itself a challenge. Tumours can also inhibit some immune responses by applying the brakes to full T cell immune activation (the basis of checkpoint inhibitor therapy). So it can be tricky for our T cells to get going or, if they do, stay active.
One way to give T cells a helping hand is to try and train T cells to find the tumours and we do this therapeutically using something called CAR T cells. The CAR T cells are made from a patient’s own immune cells. The T cells are genetically modified so that they can recognize and attack specific tumour features or antigens — we call these chimeric antigen receptors (CARs). CARs are found on the tumours and are typically common antigens in that tumour. You can then grow the cells up, put the cells back in the patient and let the CAR T cells find and eliminate their targets once they are back in the patient.
Car T cells work really well in blood cancers like some leukaemias and lymphomas but they have not worked so well in solid tissue tumours. One reason they may be less effective is that the T cells can become exhausted- this is not quite the same as us doing a work out though! T cell exhaustion is an important protective concept to prevent unwanted immune responses that can occur once a threat has been cleared. Another issues is variation in just how well some patients do with CAR T cell therapy.
Researchers compared patients who had responded well to CAR T therapy for their blood cancer with those who had not. The scientists compared the profiles of the CAR T cells in the two groups of patients focusing in on things that regulate T cell activity. So called “Good responders” had evidence of just over 40 genes being more activate. All these genes were regulated by one master-switch protein called FOXO1. They then changed the CAR T cells to make them have more FOXO1 and the cells now looked different- more like something called a T memory cell. Memory cells are long lived cells that remember threats and can be quickly deployed to deal with them should the threat re-emerge. Experiments in mice showed that the extra FOXO1 made the CAR T cells better at killing blood cancers and even more excitingly - solid tumours. This could be a game changer if the finding translates into humans.
The best ideas often come from more than one source and this exciting finding was confirmed in a separate study which used a completely different approach but came to the same conclusion! They were looking at the role of a cytokine called Interleukin-15 (IL-15). Interleukins are small soluble proteins that act as important signals for cells in our body- helping promote cellular function. IL-15 acts, amongst other things, as a growth factor that can sustain T memory cell survival. By investigating the impact of IL-15 on T cells they discovered that IL-15 upregulated expression of…. you guessed it…. FOXO1! Further lab studies verified this exciting discovery.
These two independent studies could reveal clues to how we can better turbo charge our immune cells to kill cancers.Immunology has increasingly become a huge player in our ability to cater treatments more tightly to individual needs and kill tumours in much more direct ways. I look forward to seeing the next exciting developments in this field.

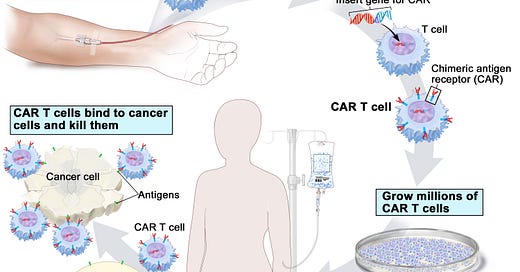


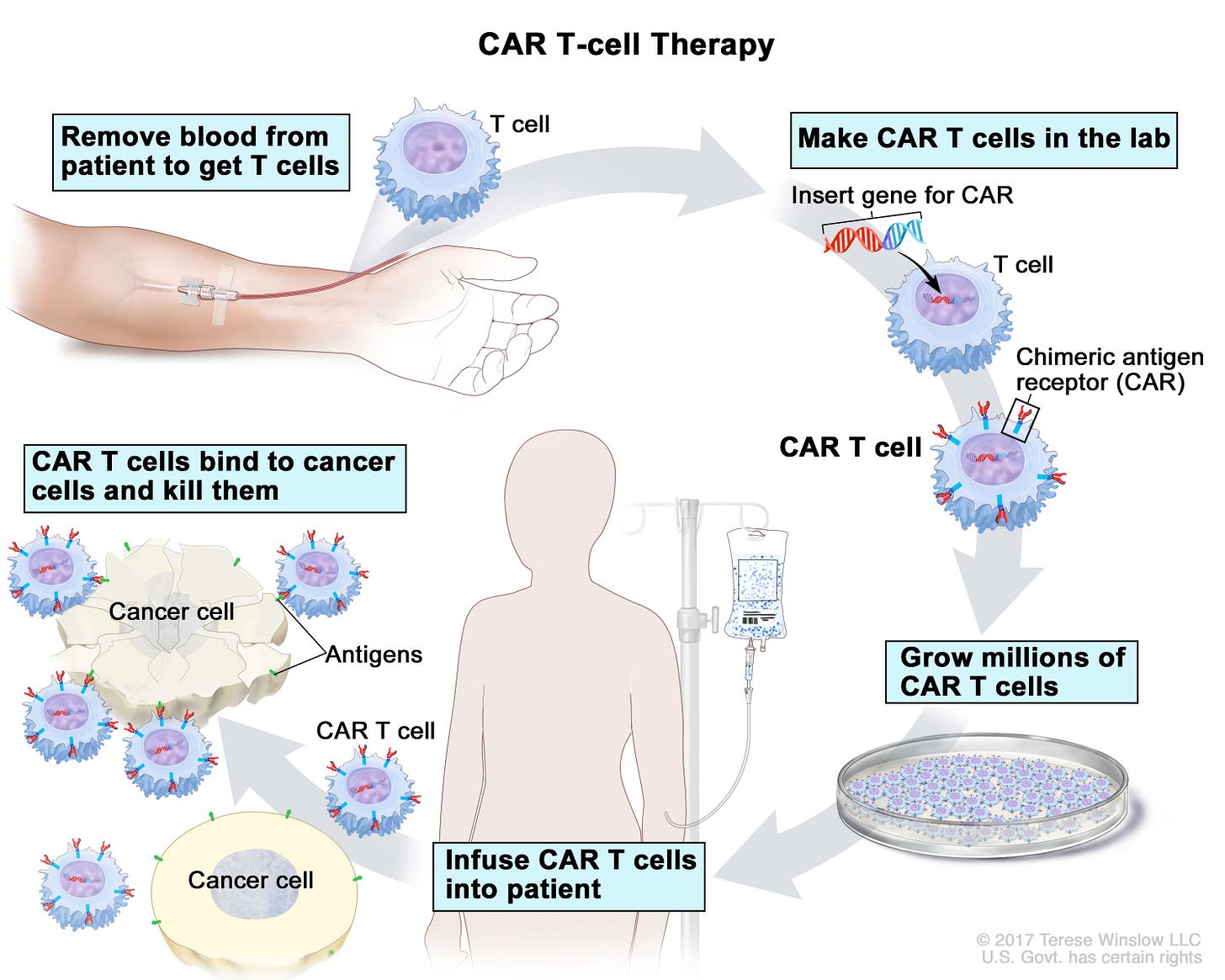
This is really interesting and a positive sign. I worry that not enough funding is going into research and development and therefore these medical advances could be hampered.